Abstract
A previously symptomless 86-year-old man received the first dose of the BNT162b2 mRNA COVID-19 vaccine. He died 4 weeks later from acute renal and respiratory failure. Although he did not present with any COVID-19-specific symptoms, he tested positive for SARS-CoV-2 before he died. Spike protein (S1) antigen-binding showed significant levels for immunoglobulin (Ig) G, while nucleocapsid IgG/IgM was not elicited. Acute bronchopneumonia and tubular failure were assigned as the cause of death at autopsy; however, we did not observe any characteristic morphological features of COVID-19. Postmortem molecular mapping by real-time polymerase chain reaction revealed relevant SARS-CoV-2 cycle threshold values in all organs examined (oropharynx, olfactory mucosa, trachea, lungs, heart, kidney and cerebrum) except for the liver and olfactory bulb. These results might suggest that the first vaccination induces immunogenicity but not sterile immunity.
Keywords: SARS-CoV-2, Vaccine, Autopsy, Histology, RT-PCR
We report on an 86-year-old male resident of a retirement home who received vaccine against SARS-CoV-2. Past medical history included systemic arterial hypertension, chronic venous insufficiency, dementia and prostate carcinoma. On January 9, 2021, the man received lipid nanoparticle-formulated, nucleoside-modified RNA vaccine BNT162b2 in a 30 μg dose. On that day and in the following 2 weeks, he presented with no clinical symptoms (Table 1 ). On day 18, he was admitted to hospital for worsening diarrhea. Since he did not present with any clinical signs of COVID-19, isolation in a specific setting did not occur. Laboratory testing revealed hypochromic anemia and increased creatinine serum levels. Antigen test and polymerase chain reaction (PCR) for SARS-CoV-2 were negative.
Table 1
Summary of major features of the patient’s history, clinical symptoms and laboratory findings, including SARS-CoV-2 testing (reference values given in brackets).
|
Day 1 |
Day 15 |
Day 18 |
Day 19 |
Day 20 |
Day 23 |
Day 24 |
Day 25 |
Day 26 |
|
|
Major event |
Vaccination |
Collapse during breakfast |
Admission to hospital; gastroscopy (mild gastritis) |
Abdominal ultrasound, initiating intravenous iron application |
Colonoscopy (ischemic colitis), initiating mesalazine |
Acute renal insufficiency, initiating intravenous glucose application |
Patient in same hospital room has positive SARS-CoV-2 RT-PCR test (Ct, 15) |
Patient somnolent, initiating antibiotic therapy, chest radiograph with minimal infiltrates |
Death at 14:30 |
|
Leading clinical symptoms |
No relevant symptoms recorded |
No further relevant symptoms recorded |
Diarrhea |
Anemia |
Anemia |
Lung auscultation with any pathological signs, hypernatremia |
Hypernatremia |
Dehydration, lung auscultation with crackles |
Acute renal and respiratory failure |
|
Temperature (°C) |
Not recorded |
Not recorded |
36.4 |
Not recorded |
Not recorded |
36.8 |
36.2 |
38.8 |
Not recorded |
|
Blood pressure (mmHg) |
Not recorded |
130/70 |
187/83 |
Not recorded |
Not recorded |
180/80 |
166/73 |
160/80 |
Not recorded |
|
Oxygen saturation (SpO2) |
Not recorded |
Not recorded |
97% |
Not recorded |
Not recorded |
Not recorded |
Not recorded |
97% + 2l O2 |
Not recorded |
|
SARS-CoV-2 test |
No data |
No data |
Antigen-test: negative |
No data |
PCR-test: negative |
No data |
No data |
RT-PCR-test: positive (Ct, 20) |
No data |
|
White-cell count (4–9/nl) |
No data |
No data |
6.6 |
7.1 |
12.1 |
13.5 |
No data |
9.2 |
15.2 |
|
Platelet count (140–400/nl) |
No data |
No data |
267 |
263 |
262 |
254 |
No data |
204 |
196 |
|
Hemoglobin (14.0–18.0 g/dl) |
No data |
No data |
7.4 |
7.1 |
7.2 |
8.0 |
No data |
8.6 |
9.3 |
|
Lactate dehydrogenase (135–250 U/L) |
No data |
No data |
179 |
165 |
No data |
No data |
No data |
No data |
439 |
|
Creatinine (0.7–1.2 mg/dl) |
No data |
No data |
1.91 |
1.78 |
No data |
2.04 |
No data |
2.17 |
3.23 |
|
C-reactive protein (< |
No data |
No data |
1.0 |
0.8 |
No data |
2.0 |
No data |
No data |
8.8 |
|
Sodium (135–145 mmol/l) |
No data |
No data |
138 |
138 |
No data |
154 |
155 |
No data |
156 |
RT-PCR, real-time polymerase chain reaction; Ct, cycle threshold.
Gastroscopy and colonoscopy were performed to investigate the cause of diarrhea further. Colonoscopy, in particular, demonstrated an ulcerative lesion of the left colonic flexure, which was histologically diagnosed as ischemic colitis. PCR-analysis on biopsy specimens, following a previously reported method (Kaltschmidt et al., 2021), was negative for SARS-CoV-2. Treatment was supportive with mesalazine and intravenous iron substitution. Subsequently, the patient’s condition deteriorated under the development of renal insufficiency. On day 24, a patient in the same hospital room as our case tested positive for SARS-CoV-2. On day 25, our patient tested SARS-CoV-2 positive by real-time PCR (RT-PCR), with a low cycle threshold (Ct) value indicating high virus load. On further analysis of the swab sample, there was no evidence for mutant SARS-CoV-2 variants B.1.1.7, B.1.351 or B.1.1.28.1. Taken together, it appears the patient became infected from the patient in his hospital room. Our patient now presented with fever and respiratory discomfort, and lung auscultation displayed crackles. Despite starting supplemental oxygen (2 l per minute) and antibiotic therapy by ceftriaxone, the patient died from acute renal and respiratory failure on the following day.
Immunogenicity assessment by measuring spike protein (S1) antigen-binding immunoglobulin (Ig) G in the serum samples obtained at day 25 showed antibody response (8.7 U/ml, reference value <0.8–1.2 U/ml; Roche ECLIA™), while (nucleocapsid) NCP-IgG/IgM was not elicited (<0.1 U/ml, reference value >1.0 U/ml; Roche ECLIA™). These results indicate that the patient had already developed relevant immunogenicity through vaccination.
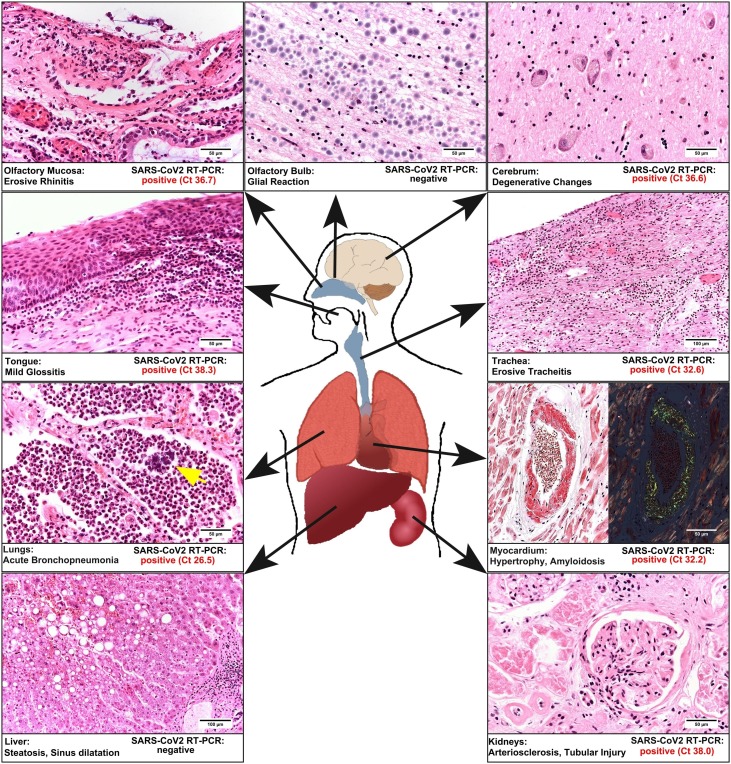
Postmortem study revealed acute bilateral bronchopneumonia with abscesses, sometimes being surrounded by bacterial cocci (Figure 1 ). There were no findings of commonly described manifestations of COVID-19-associated pneumonitis. In the heart, we found biventricular hypertrophy (weight 580 g) and histologically, we diagnosed ischemic cardiomyopathy. We detected amyloidosis of the transthyretin type in the heart and to a lesser extent in the lungs. The kidneys revealed both chronic damage with arteriolosclerosis and interstitial fibrosis, and acute renal failure with hydropic tubular degeneration. The examination of the brain revealed a left parietal pseudocystic tissue necrosis, which was diagnosed as an old infarction area.
Synopsis of the relevant histological findings and the results of molecular mapping is presented. The histomorphology is obtained by standard hematoxylin and eosin reaction, except for the myocardium on the right side (Congo red staining). The magnification is shown by bars. Note that in the lungs, we also observed colonies of cocci (arrow) in granulocytic areas. In addition, the results of molecular mapping are given as evaluated cycle threshold values of the real-time polymerase chain reaction for SARS-CoV-2. Note that only in the olfactory bulb and the liver SARS-CoV-2 could not be detected.
We conducted molecular mapping of 9 different anatomical parts of formalin-fixed paraffin-embedded tissue as previously described (Kaltschmidt et al., 2021). RNA was extracted from paraffin sections using the Maxwell RSC (Promega, Madison, WI, USA). Multiplex RT-PCR analysis targeted 2 independent genes of the SARS-CoV-2-genome (Fluorotype SARS-CoV-2 plus Kit; HAIN/Bruker, Nehren, Germany): RNA-dependent RNA polymerase (Target 1) and nucleopeptide (Target 2). The negative cut-off value was Ct >45. We examined 9 different tissue samples for known and relevant pathways of virus spreading in the human body (Figure 1). To prevent cross-contamination, each specimen was directly embedded in separate tissue cassettes and separately fixed in 4% phosphate-buffered saline-buffered formalin. We demonstrated viral RNA in nearly all organs examined except for the liver and the olfactory bulb (Figure 1).
A detailed autopsy study including molecular virus mapping of a patient vaccinated against SARS-CoV-2 with a positive SARS-CoV-2 test post-vaccination has not previously been reported, to the authors’ knowledge. We suggest that a single treatment with BNT162b2 RNA vaccine elicited significant immunogenicity, as reflected in the reported spike protein-based neutralizing IgG serum values. From the weeks before vaccination, through vaccination (day 1), to shortly before death (day 24), the patient was free of any clinical symptoms typically ascribed to COVID-19. Furthermore, blood work did not show an IgM titer that is generally observed 7–14 days after symptom onset (Kim et al., 2020). However, the patient tested SARS-CoV-2 positive. Both the Ct value measured in nasopharyngeal swab and values measured in formalin-fixed paraffin-embedded autopsy specimens indicate viral load and suggest transmissibility. Because our patient died approximately 2 days after his first positive SARS-CoV-2 test result, we suppose that the molecular mapping data reflects an early stage of viral infection. An early stage of infection might also explain why different regions such as the olfactory bulb and liver were not (yet) affected by systemic viral spread.
We did not observe any characteristic morphological features of COVID-19 reported in comprehensive morphological autopsy studies so far (Schaller et al., 2020, Edler et al., 2020, Ackermann et al., 2020). We did not find any typical signs of diffuse alveolar damage in the lungs, but we identified extensive acute bronchopneumonia, possibly of bacterial origin. We concluded that the patient died from bronchopneumonia and acute renal failure.
Our findings are in line with previous evidence from animal models that immunization against SARS-CoV-2 by vaccination appeared to reduce the severity of pathogenesis, especially with regard to severe lung disease, while viral RNA persisted in nasal swabs (Van Doremalen et al., 2020, Vogel et al., 2021). Recently, Amit et al. (2021) published results on a clinical trial on healthcare workers using vaccine BNT162b2 that demonstrated substantial early reductions in SARS-CoV-2 infection and symptomatic COVID-19 rates following administration of the first vaccine dose.
Concerning major adverse effects in patients receiving vaccination against SARS-CoV-2, local effects predominate, and severe systemic reactions are rarely described (Yuan et al., 2020). However, recent reports of an increased risk of blood clots, particularly of cerebral venous sinus thrombosis in the case of the Oxford-AstraZeneca vaccine (Mahase 2021), raised a matter of debate on the safety of COVID-19 vaccine in general. Comprehensive analysis of autopsy data must be performed to provide more detailed insights into lethal adverse effects and any deaths associated with vaccination.
In summary, the results of our autopsy case study in a patient with mRNA vaccine confirm the view that by first dose of vaccination against SARS-CoV-2 immunogenicity can already be induced, while sterile immunity is not adequately developed.
Conflicts of interest
The authors do not have any commercial or financial conflict of interest.
Ethical approval
This case study was performed in the setting of the German national “Defeat Pandemics” project, approved by the Medical Association of Westphalia-Lippe, Münster, Germany (Ref. 2020-575-b-S) and carried out in accordance with the ethical principles of the Helsinki Declaration. Informed consent by the next-of-kin was available.
Funding source
There was no funding received from any individual or organization.
Acknowledgements
We are grateful for the expert technical assistance of Ralf Bode and Nadine Weber (University Hospital of OWL of the University of Bielefeld, Campus Lippe, Detmold).
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV2-infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–877. doi: 10.1016/S0140-6736(21)00448-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Edler C., Schröder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F. Dying with SARS-CoV2 infection – an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02336-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kaltschmidt B., Fitzek A.D.E., Schaedler J., Förster C., Kaltschmidt C., Hansen T. Hepatic vasculopathy and regernative responses of the liver in fatal cases of COVID-19. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.01.044. In press. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim D.S., Rowland-Jones S., Gea-Mallorqui E. Will SARS-CoV-2 infecion elicit long-lasting protective or sterilizing immunity? Implications for vaccine strategies. Front Immunol. 2020;11:571481. doi: 10.3389/fimmu.2020.571481.eCollection2020. [PMC free article] [PubMed] [CrossRef – https://dx.doi.org/10.3389%2Ffimmu.2020.571481.eCollection2020] [Google Scholar]
- Mahase E. Covid-19: AstraZeneca vaccine is not linked to increased risk of blood clots, finds European Medicine Agency. BMJ. 2021;372:n774. doi: 10.1136/bmj.n774. [PubMed] [CrossRef] [Google Scholar]
- Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B. Postmortem examinations of patients with COVID19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Van Doremalen N., Lambe T., Spencer A., Belij-Rammersdorfer S., Purushotham J.N., Port J.R. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1101/2020.05.13.093195. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1101/2020.12.11.421008. [PubMed] [CrossRef] [Google Scholar]
- Yuan P., Ai P., Liu Y., Ai Z., Wang Y., Cao W. Safety, tolerability, and immunogenicity of COVID19 vaccines: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.11.03.20224998. Preprint. [CrossRef] [Google Scholar]
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8051011/